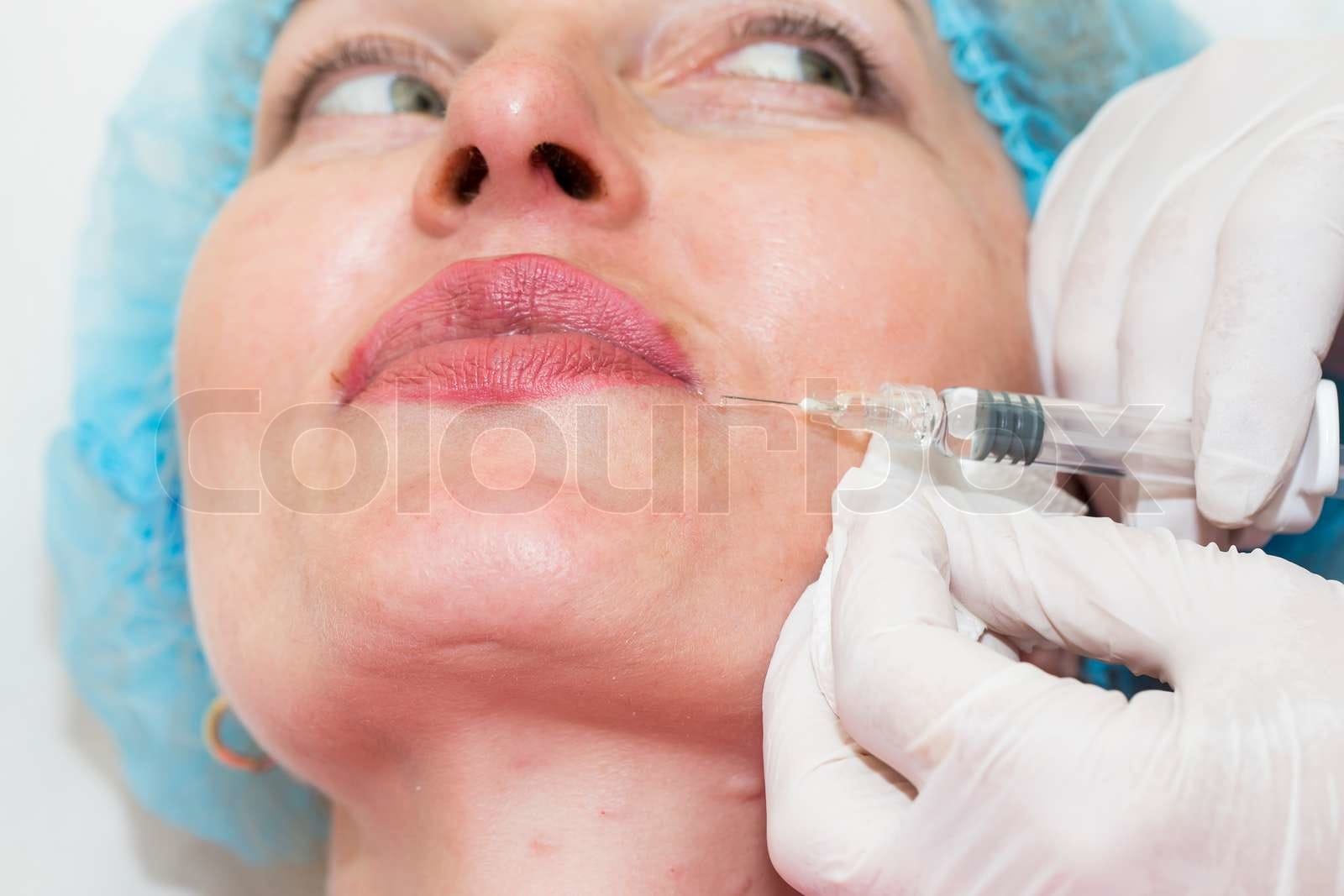
In recent weeks, health officials have initiated an investigation concerning several potentially fatal cases of botulism that have been linked to cosmetic injections, particularly those involving botulinum toxin, commonly referred to by its brand name, Botox. The incidents, which have raised significant concern among medical professionals and patients alike, highlight a pressing need for stricter regulation in the booming cosmetic enhancement market.
Botulism is a rare but serious illness caused by a toxin produced by the bacterium Clostridium botulinum. This toxin attacks the nervous system and can result in paralysis, respiratory failure, and in extreme cases, death. Patients who have developed symptoms of botulism after cosmetic procedures have exhibited a range of complications, most notably muscle weakness, difficulties in swallowing, and respiratory distress. Responding to the alarming reports, health authorities have emphasized the importance of recognizing the signs of botulism and the need for immediate medical attention to mitigate potential complications.
Many of the reported cases have involved injections that were not administered by licensed or properly trained professionals, including situations where patients sought treatments in unregulated environments. This has once again sparked debate regarding the legal and ethical implications surrounding the cosmetic injection industry, which often operates in a gray area of regulation. The investigation aims to trace back these cases to their origins, examining both the qualifications of practitioners and the sourcing of the products used in these procedures.
The Centers for Disease Control and Prevention (CDC), along with various state health departments, is actively working to determine the scope of the issue. They are coordinating with hospitals where patients have been treated to collect crucial data on the incidents. Furthermore, they are advising individuals who have recently undergone such procedures to seek medical help if they experience any symptoms indicative of botulism. The CDC also encourages those considering cosmetic injections to conduct thorough research, ensuring their procedures are performed in certified medical facilities by experienced and licensed providers.
One of the ongoing concerns is the commercialization of cosmetic treatments via social media platforms, which often serve as advertising channels for unregulated providers. The allure of relatively cheap pricing can lead individuals to make impulsive decisions without fully understanding the potential risks involved. Several cases under investigation have emerged from providers who have misrepresented their qualifications or the safety of the products they are using.
As medical professionals and regulatory bodies assess the current state of the cosmetic injection industry, experts emphasize the importance of transparency and accountability. A growing number of practitioners advocate for mandatory certifications and standardized training for all individuals performing cosmetic procedures. Only through enhanced regulations can patient safety be prioritized in an industry that faces continual growth amid rising demand.
The urgency surrounding this investigation reflects a broader concern about patient safety in cosmetic procedures. While many individuals undergo cosmetic injections without complications, the increasing number of botulism cases serves as a stark reminder of the serious implications associated with such treatments. This incident underscores the necessity for patients to be fully informed regarding their options and the inherent risks involved with cosmetic procedures.
In light of recent events, some states are re-evaluating their policies regarding the regulation of cosmetic injections. Potential legislative changes aimed at tightening controls on who can perform these procedures and what products can be used are being considered as health departments work toward safeguarding the public.
In conclusion, the investigation into potentially fatal botulism cases linked to cosmetic injections is prompting a reevaluation of safety standards within the industry. As health authorities continue to investigate, it is crucial for patients to exercise caution, ensuring that they seek treatment only from credible, licensed professionals in accredited facilities. The findings from this ongoing investigation may lead to significant changes in regulatory practices, emphasizing the importance of safeguarding public health in the rapidly evolving landscape of cosmetic enhancements.