The FDA has approved Merck’s respiratory syncytial virus (RSV) vaccine for infants, a significant development that heightens competition with existing products from Sanofi and AstraZeneca. This approval opens new avenues in the fight against RSV, particularly among vulnerable infant populations.
Tag: Vaccine
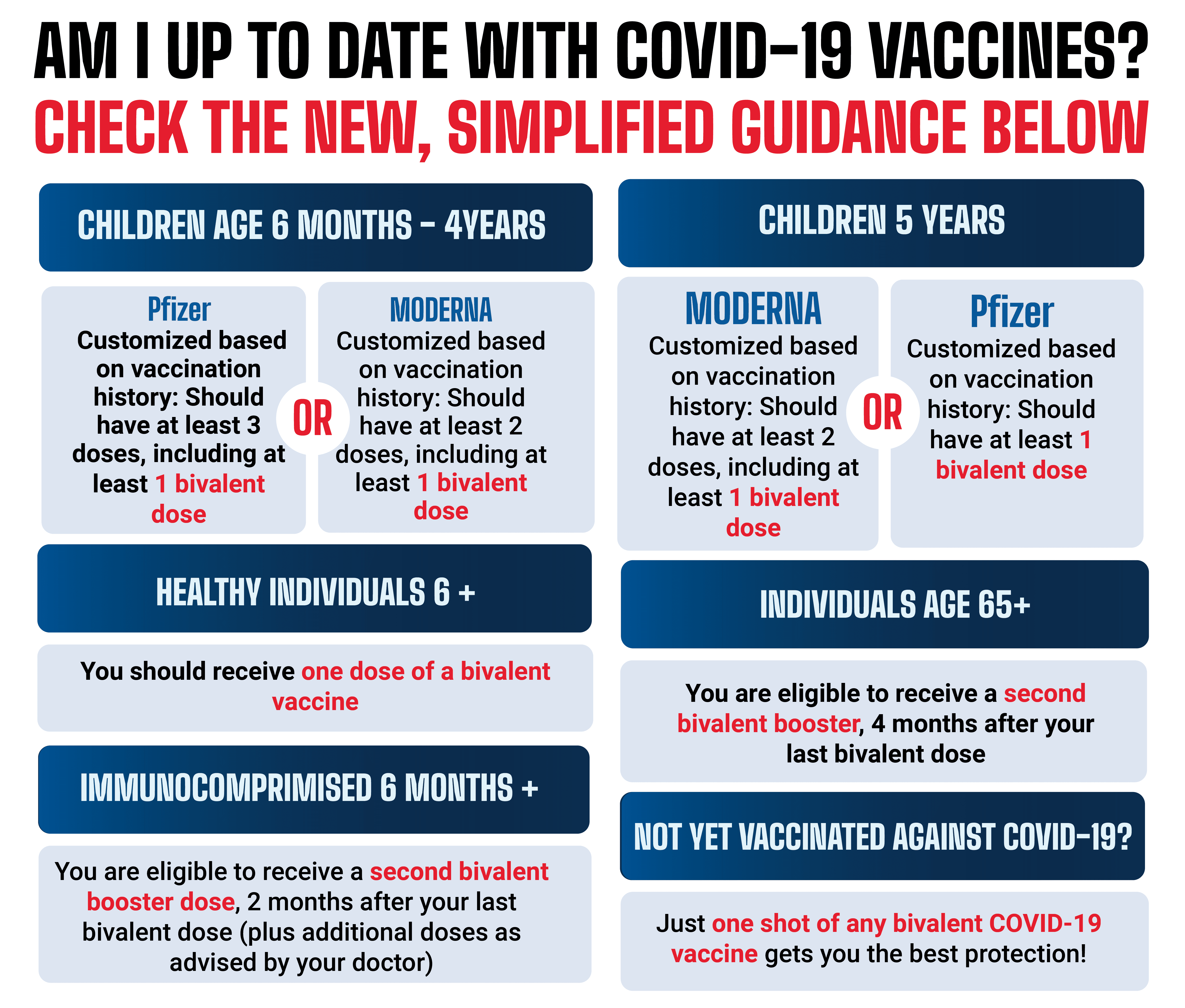
FDA Greenlights Moderna’s New Lower-Dose COVID-19 Vaccine
The FDA has approved Moderna’s latest lower-dose COVID-19 vaccine, aimed at enhancing accessibility and compliance for vaccination. This decision reflects ongoing efforts to combat the pandemic and improve public health outcomes.
Stanford Scientists Identify New COVID Variant Amidst U.S. Vaccine Accessibility Changes
Stanford scientists have detected a new COVID variant in California, raising concerns as the U.S. government implements stricter regulations on vaccine access. This development highlights ongoing challenges in the fight against the pandemic and calls for renewed public health strategies.
Moderna Receives Federal Funding to Expedite Avian Flu Vaccine Development
The U.S. Department of Health and Human Services (HHS) has awarded Moderna $590 million to accelerate the development and clinical trials of a vaccine targeting avian influenza, commonly known as bird flu. This funding aims to expedite the availability of a vaccine to combat potential future outbreaks, especially given the ongoing concerns about the virus’s potential to jump to humans. The grant supports late-stage clinical trials and associated manufacturing enhancements.
Moderna’s Stock Decline Linked to Disappointing COVID-19 Vaccine Projections
Moderna’s stock experienced a significant drop today following the company’s announcement of lower-than-expected forecasts for its COVID-19 vaccine sales. The pharmaceutical company cited various factors contributing to the revised projections, including changing market dynamics and increased competition. Investors reacted swiftly to the news, leading to a notable decline in share prices.
Understanding the HMPV Outbreak in China: Insights and Implications
The recent outbreak of Human Metapneumovirus (HMPV) in China has raised questions regarding its nature, similarities to COVID-19, and potential vaccine availability. HMPV, while not a new virus, shares some characteristics with COVID-19, including respiratory symptoms and transmission dynamics. This article explores what is known about HMPV, its impact, and the current state of vaccine development.
HMPV Outbreak in China: Understanding a New Viral Threat
The recent outbreak of Human Metapneumovirus (HMPV) in China has raised concerns among health authorities and the general public. Similar to COVID-19, HMPV is a respiratory illness that has garnered attention due to its potential to spread quickly and cause severe illness, particularly in vulnerable populations. However, not much is known about the virus yet, and research is ongoing to develop vaccines and treatments. This article will delve into the details of the HMPV outbreak, its similarities to COVID-19, the current status of vaccines, and the latest information available.Category: HealthTags: Human Metapneumovirus, HMPV, China, COVID-19, Virus, VaccineSticky: TrueImage: HMPV_outbreak
Local West Michigan Doctor Takes Precautions as Norovirus Cases Increase
A West Michigan doctor is taking action to prevent the spread of the highly contagious Norovirus as cases arise across the area.
Breaking News: HMPV Outbreak in China – Facts and Updates
Health authorities in China are closely monitoring a new respiratory virus, labeled as HMPV, which has been detected in multiple provinces across the country. The virus appears to share similarities with Covid-19 in terms of its mode of transmission and severity of symptoms. Scientists and researchers are working diligently to figure out the extent of the outbreak, how it spreads, and whether a vaccine is currently available. This article will provide an overview of the latest developments related to the HMPV outbreak in China.