The FDA has announced the early rollout of its new AI tool agencywide, aiming to enhance its regulatory processes and improve public health outcomes. This initiative, now introduced weeks ahead of its original schedule, marks a significant step in the agency’s efforts to integrate advanced technology in its operations and decision-making.
Tag: FDA
FDA Greenlights Moderna’s New Lower-Dose COVID-19 Vaccine
The FDA has approved Moderna’s latest lower-dose COVID-19 vaccine, aimed at enhancing accessibility and compliance for vaccination. This decision reflects ongoing efforts to combat the pandemic and improve public health outcomes.
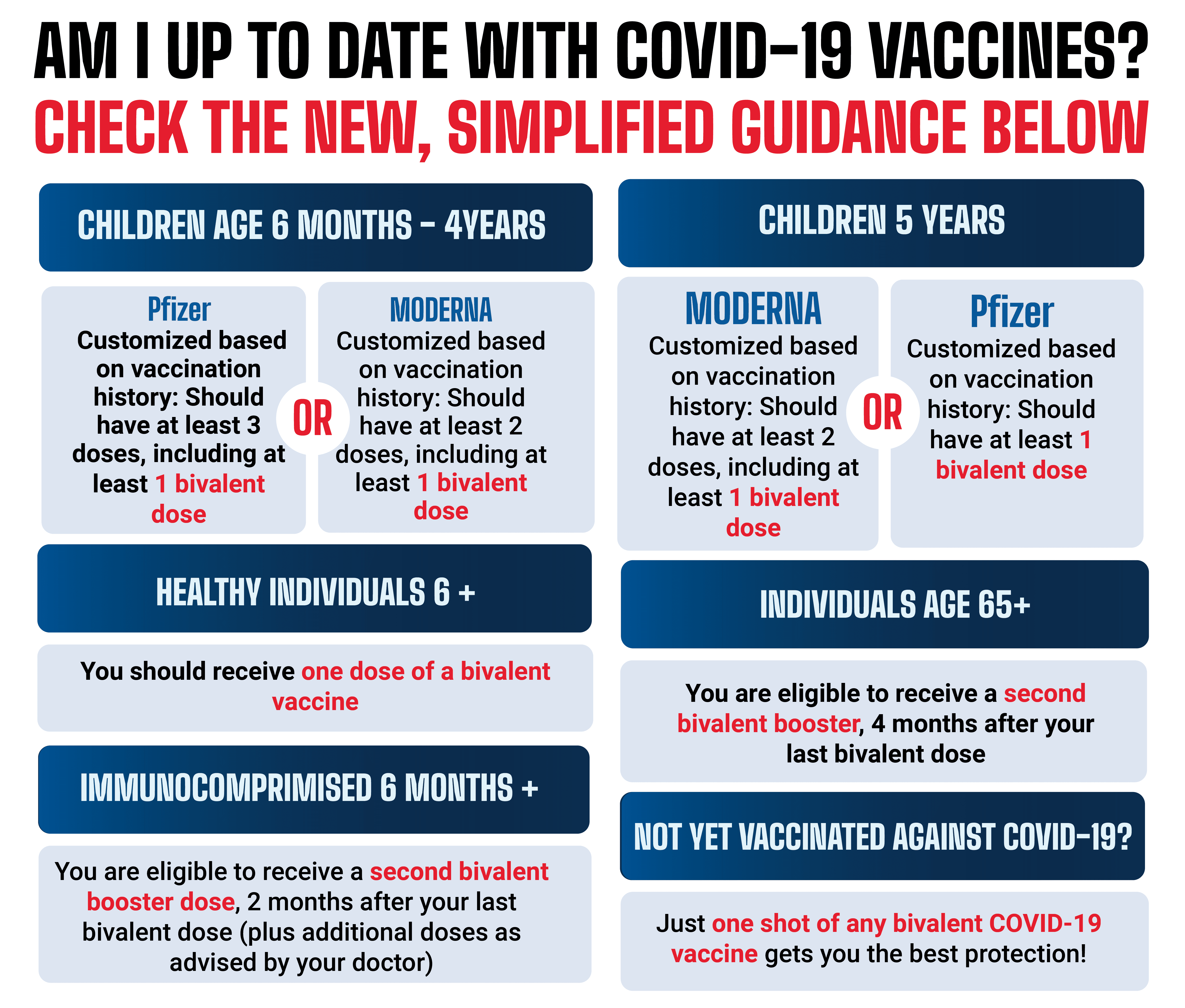
FDA’s Vaccine Limitation Proposal Raises Concerns Among Ineligible Groups
The FDA’s recent proposal to limit eligibility for COVID-19 vaccines has sparked apprehension among certain groups who may no longer qualify for vaccination. This article explores the implications of the FDA’s plan and its potential impact on public health.
Breakthrough Approval: First Blood Test for Alzheimer’s Disease Diagnosis
The FDA has announced the approval of a groundbreaking blood test designed to aid in the diagnosis of Alzheimer’s disease. This novel test offers a minimally invasive option for identifying biomarkers related to the disease, which may enhance early diagnosis and treatment strategies, paving the way for advancements in Alzheimer’s care.
FDA Grants Approval for Groundbreaking At-Home Cervical Cancer Screening Device
The FDA has approved a new at-home cervical cancer screening device, marking a significant advancement in women’s health care. Developed by a leading medical technology company, this device enables women to conduct screenings privately and conveniently, potentially increasing early detection rates of cervical cancer.
FDA Appoints Oncologist Vinay Prasad as New Chief of Vaccines
The FDA has officially announced the appointment of oncologist Vinay Prasad as its new chief of vaccines. Prasad, known for his contributions to cancer research and public health, will lead vaccine strategy amid ongoing public health challenges.
FDA Official Brenner Delays Decision on Novavax COVID-19 Vaccine
Dr. Peter Brenner, a top official at the FDA, has decided to pause the evaluation of the Novavax COVID-19 vaccine as concerns about its safety and efficacy arise. This decision underscores ongoing scrutiny within vaccine approvals as public health authorities aim to maintain safety standards.
FDA Grants Approval for Johnson & Johnson’s Nasal Spray as a Standalone Treatment for Depression
The U.S. Food and Drug Administration (FDA) has officially approved Johnson & Johnson’s nasal spray, Spravato, as a standalone treatment for depression. This decision marks a significant advancement in the management of major depressive disorder, particularly for patients who have not responded to traditional therapies. The approval is based on clinical trials demonstrating the efficacy and safety of the treatment, which offers a new option for individuals struggling with this debilitating condition.
FDA Grants Approval for Johnson & Johnson’s Nasal Spray as a Standalone Treatment for Depression
The U.S. Food and Drug Administration (FDA) has officially approved Johnson & Johnson’s nasal spray, Spravato, as a standalone treatment for depression. This decision marks a significant advancement in the management of major depressive disorder, particularly for patients who have not responded to traditional therapies. The approval is based on clinical trials demonstrating the spray’s efficacy and safety, providing a new option for those struggling with this debilitating condition.
Raw Milk Brand Chief Claims Recall is Politically Motivated Amid Possible FDA Role
The head of a popular raw milk brand has stirred controversy by alleging that the recent recall of its products is politically motivated, a claim that comes as he is rumored to be considered for a role within the FDA.