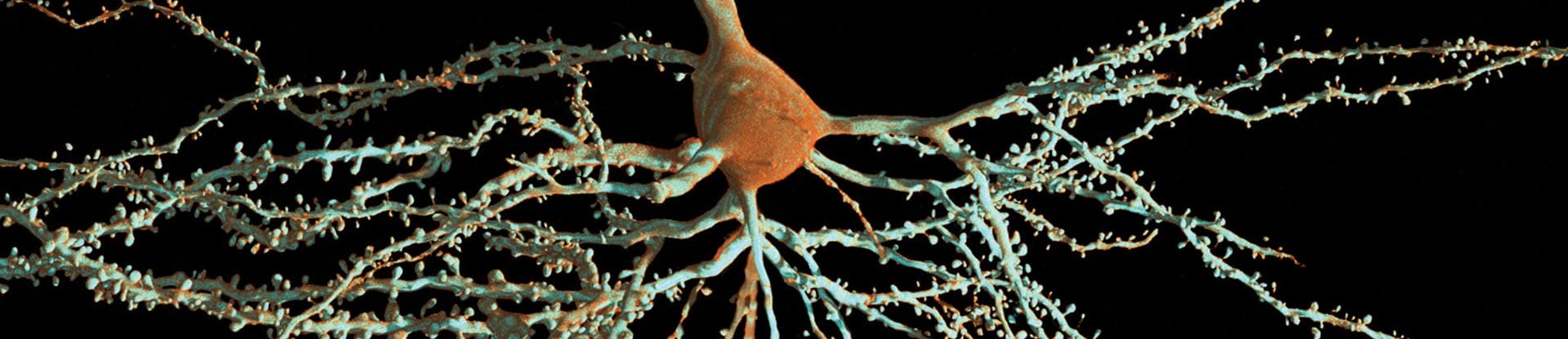
The aging process is a complex phenomenon that affects various systems within the human body, with the brain being one of the most significantly impacted. Recent scientific advancements have shed light on the cellular mechanisms underlying brain aging, revealing critical insights into how age-related damage occurs at the molecular level. Understanding these processes is essential for developing strategies to promote healthy aging and combat cognitive decline.
One of the primary findings of recent research is the role of cellular senescence in brain aging. Cellular senescence refers to the state in which cells lose their ability to divide and function properly, often as a result of stress or damage. In the context of the brain, senescent cells can accumulate over time, leading to a decline in neuronal function and contributing to the overall aging of the brain. These senescent cells release various pro-inflammatory factors, collectively known as the senescence-associated secretory phenotype (SASP), which can further exacerbate inflammation and damage to surrounding healthy cells.
Moreover, researchers have identified specific proteins and pathways that are implicated in the aging process. For instance, the protein p53, known for its role in tumor suppression, has also been linked to the regulation of cellular senescence. In aged brains, elevated levels of p53 can lead to increased senescence and neuronal loss. This connection underscores the importance of p53 as a potential target for therapeutic interventions aimed at mitigating age-related cognitive decline.
Another significant aspect of brain aging involves the accumulation of damaged proteins and organelles within neurons. As individuals age, the efficiency of cellular repair mechanisms tends to decline, leading to the buildup of misfolded proteins and dysfunctional mitochondria. This accumulation can disrupt cellular homeostasis, resulting in impaired energy production and increased oxidative stress. Oxidative stress, in turn, contributes to neuronal injury and death, further advancing the aging process.
Research has also highlighted the role of neuroinflammation in brain aging. The brain’s immune cells, known as microglia, become increasingly activated as individuals age. While microglia play a crucial role in maintaining brain health by clearing debris and responding to injury, their chronic activation can lead to sustained inflammation. This neuroinflammatory state is associated with various neurodegenerative diseases, including Alzheimer’s disease and Parkinson’s disease. Understanding the triggers of microglial activation and the subsequent inflammatory responses is crucial for developing strategies to protect the aging brain.
Another key player in brain aging is the decline in neurogenesis, the process by which new neurons are generated from neural stem cells. Neurogenesis primarily occurs in the hippocampus, a region of the brain associated with learning and memory. Research indicates that neurogenesis declines with age, which may contribute to memory deficits commonly observed in older adults. Factors such as stress, inflammation, and changes in hormonal levels can negatively impact neurogenesis, highlighting the importance of maintaining a supportive environment for neural stem cells as individuals age.
Recent studies have also explored the potential of lifestyle interventions to counteract the effects of brain aging. Regular physical exercise, for instance, has been shown to promote neurogenesis and enhance cognitive function in older adults. Additionally, a balanced diet rich in antioxidants and anti-inflammatory compounds may help mitigate oxidative stress and inflammation, contributing to better brain health. Such findings underscore the importance of a holistic approach to aging, incorporating both biological understanding and lifestyle modifications.
The implications of this research extend beyond understanding the aging process itself. By identifying the key cellular players and mechanisms involved in brain aging, scientists can begin to develop targeted therapies aimed at preserving cognitive function in older adults. Potential strategies may include pharmacological interventions designed to reduce cellular senescence, enhance neurogenesis, or modulate neuroinflammation. Furthermore, ongoing research into the genetic and epigenetic factors influencing brain aging may provide additional avenues for intervention.
In conclusion, the exploration of brain aging at the cellular level has revealed a complex interplay of mechanisms that contribute to age-related cognitive decline. By identifying key players such as senescent cells, inflammatory pathways, and neurogenic processes, researchers are paving the way for future interventions that may help maintain cognitive health in aging populations. Continued investigation into these cellular mechanisms will be critical for developing effective strategies to promote healthy aging and improve the quality of life for older adults.