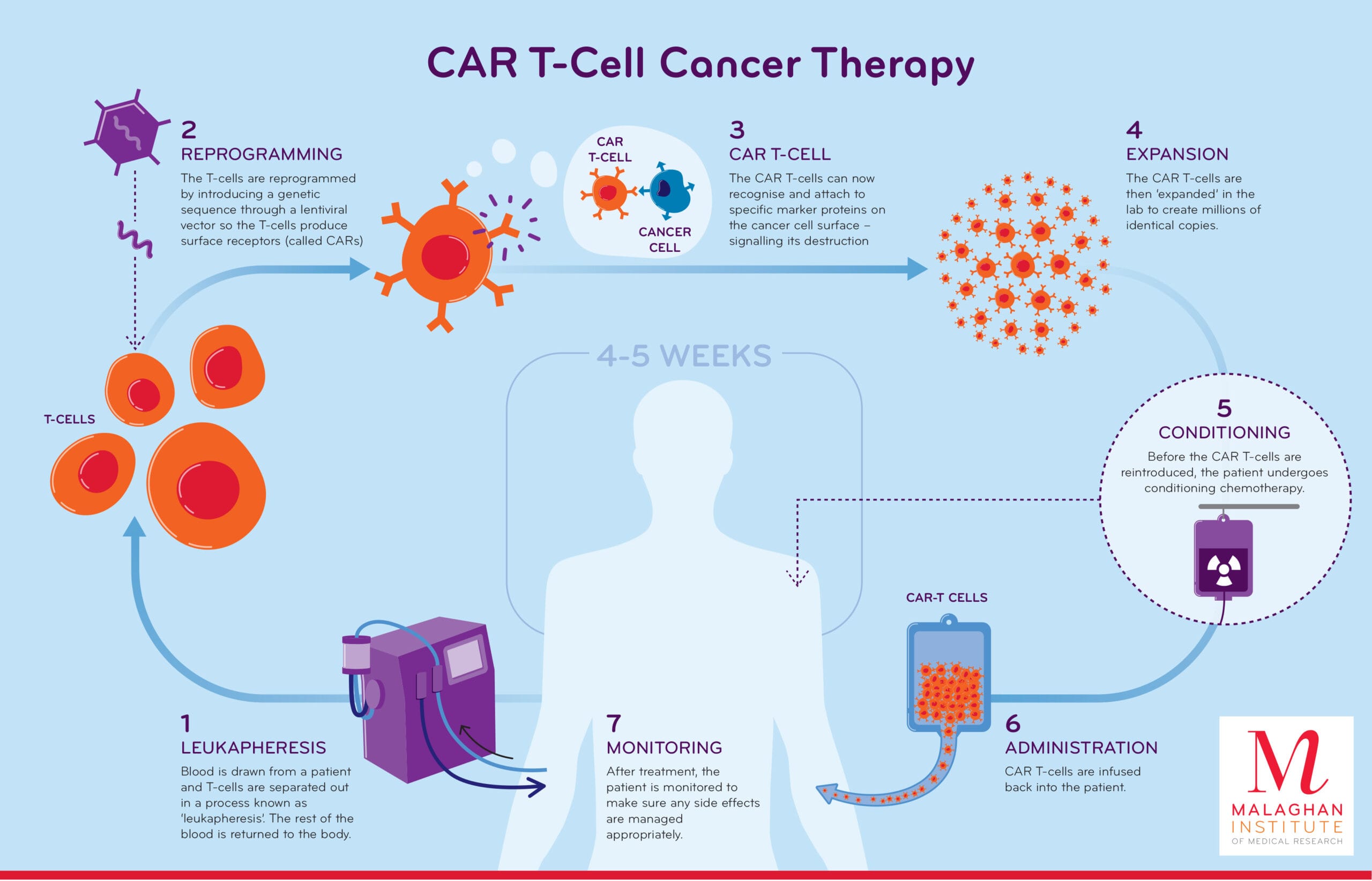
Cancer immunotherapy, particularly CAR (chimeric antigen receptor) T cell therapy, has revolutionized the treatment of various types of cancer by harnessing the body’s immune system to fight the disease. CAR T cells are engineered to recognize specific antigens on the surface of cancer cells, allowing them to target and destroy these cells more effectively. However, the effectiveness of CAR T cell therapy can be limited by several factors, including the availability of nutrients and energy sources that support the function and survival of these immune cells.
Recently, researchers have been exploring the potential of the ketogenic diet (keto diet) to enhance the efficacy of CAR T cell therapy. The keto diet is a high-fat, low-carbohydrate, moderate-protein diet that puts the body into a state of ketosis, in which the liver converts fat into molecules called ketones, which can be used as an alternative source of energy. Studies have shown that the keto diet can have anti-tumor effects and improve the outcomes of various cancer treatments, including chemotherapy and radiation therapy.
A new study published in the journal Nature Medicine has found that a metabolite produced during ketosis, called beta-hydroxybutyrate (BHB), can enhance the performance of CAR T cells against cancer. BHB is a type of ketone that is produced when the body breaks down fat for energy. The researchers discovered that BHB can increase the energy metabolism of CAR T cells, allowing them to proliferate and expand more effectively, and to produce more cytokines, which are signaling molecules that help to activate the immune response against cancer cells.
The study was conducted using mouse models of leukemia and lymphoma, in which CAR T cells were engineered to target specific antigens on the surface of cancer cells. The researchers found that mice that received CAR T cells and were fed a keto diet had significantly improved outcomes compared to those that received CAR T cells and were fed a standard diet. The keto diet was found to increase the production of BHB, which in turn enhanced the energy metabolism of CAR T cells and improved their ability to target and kill cancer cells.
The researchers also found that BHB can inhibit the activity of a protein called PD-1, which is a checkpoint protein that can suppress the activity of immune cells, including CAR T cells. By inhibiting PD-1, BHB can help to overcome immune suppression and enhance the anti-tumor activity of CAR T cells.
These findings have important implications for the development of new strategies for enhancing cancer immunotherapy. The use of the keto diet or BHB supplements could potentially be used to improve the outcomes of CAR T cell therapy, particularly in patients with aggressive or refractory cancers. Additionally, the study suggests that other metabolites produced during ketosis may also have anti-tumor effects and could be explored as potential therapeutic agents.
While the study’s findings are promising, further research is needed to confirm the safety and efficacy of using the keto diet or BHB supplements in patients with cancer. Additionally, the study’s results may not be generalizable to all types of cancer, and further research is needed to determine which types of cancer may be most responsive to this approach.
In conclusion, the study provides new insights into the potential of the keto diet to enhance the efficacy of CAR T cell therapy, and highlights the importance of further research into the use of metabolites produced during ketosis as therapeutic agents in cancer treatment.