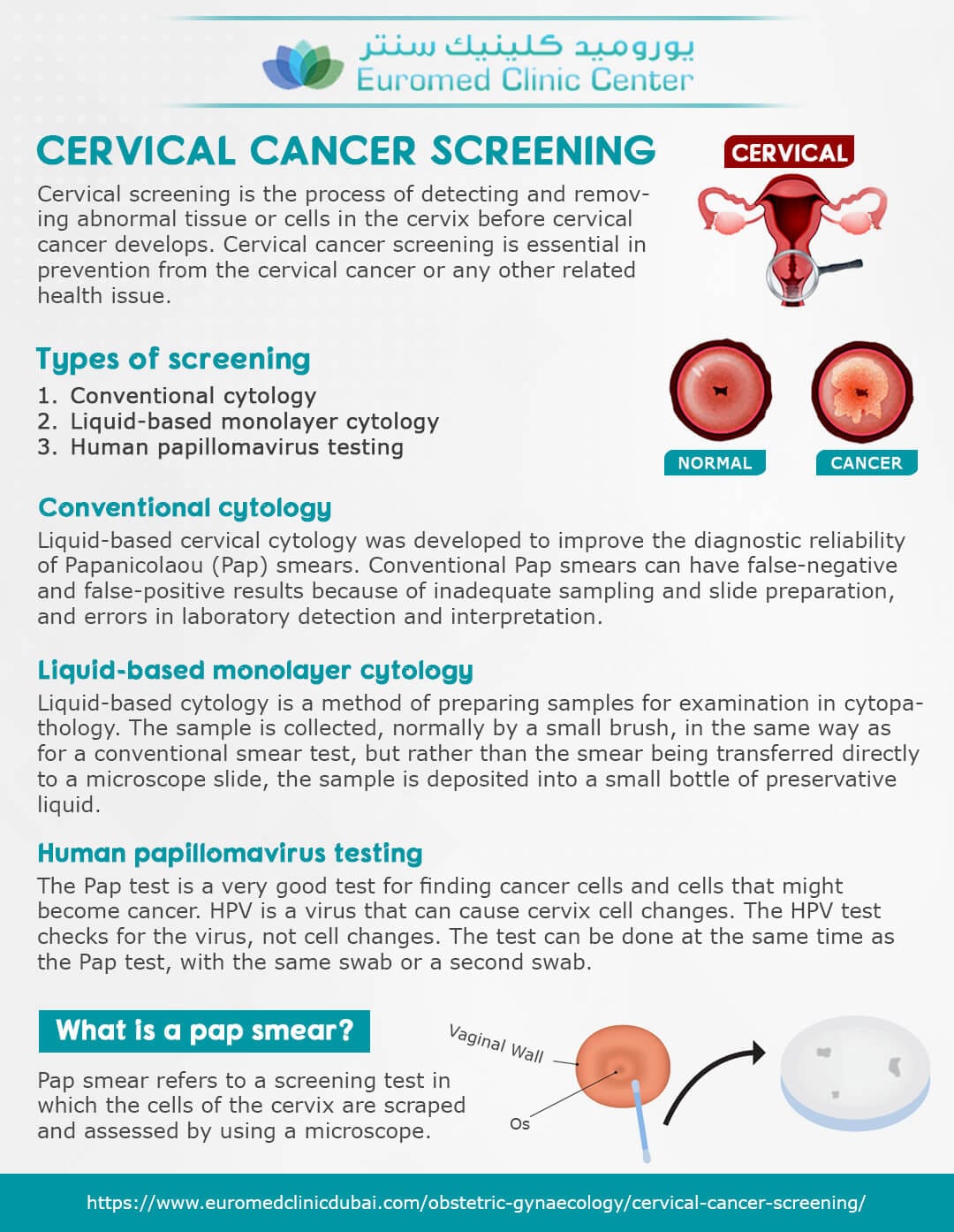
Cervical cancer is a significant public health concern, with an estimated 13,800 new cases and 4,200 deaths reported in the United States in 2020 alone. Early detection and screening are crucial in preventing cervical cancer, and the US Preventive Services Task Force (USPSTF) has been working to update its guidelines to reflect the most effective screening methods.
The USPSTF has recently drafted a new recommendation that proposes HPV testing as the preferred method for cervical cancer screening in women aged 30 and above. This shift away from Pap smears marks a significant change in the way healthcare providers approach cervical cancer screening.
HPV (human papillomavirus) is a common sexually transmitted infection that can cause cervical cancer. The virus is highly prevalent, with an estimated 79 million Americans currently infected. HPV testing involves collecting a sample of cells from the cervix, which is then tested for the presence of high-risk HPV types. This method has been shown to be more effective in detecting cervical cancer and precancerous lesions than traditional Pap smears.
The USPSTF’s draft recommendation is based on a comprehensive review of existing evidence, which suggests that HPV testing is more sensitive and specific than Pap smears in detecting cervical cancer. The review found that HPV testing can detect high-grade precancerous lesions and cervical cancer with greater accuracy than Pap smears. Additionally, HPV testing can identify women who are at risk of developing cervical cancer, allowing for earlier intervention and prevention.
The American College of Obstetricians and Gynecologists (ACOG) has also endorsed the use of HPV testing for cervical cancer screening. In a statement, ACOG noted that HPV testing is a “more efficient and effective” method than Pap smears, and that it can help reduce the number of unnecessary follow-up tests and procedures.
The shift towards HPV testing is likely to have significant implications for healthcare providers and patients. Women aged 30 and above who are due for cervical cancer screening can expect to undergo HPV testing instead of Pap smears. This change may also lead to a reduction in the number of unnecessary colposcopies and biopsies, which can be costly and invasive procedures.
However, some experts have raised concerns about the potential limitations of HPV testing. For example, HPV testing may not detect all types of cervical cancer, and false-negative results can occur. Additionally, HPV testing may not be suitable for women who have had a hysterectomy or who have a weakened immune system.
Despite these limitations, the USPSTF’s draft recommendation marks an important step forward in the fight against cervical cancer. By adopting HPV testing as the preferred method for cervical cancer screening, healthcare providers can improve early detection and prevention of this devastating disease.