The landscape of cervical cancer screening may soon be undergoing a significant shift, with a prominent health task force issuing a draft recommendation that favors primary human papillomavirus (HPV) testing over the traditional Papanicolaou (Pap) test for women aged 30 and older. This proposed change in screening protocol, if implemented, could have far-reaching implications for how healthcare providers approach cervical cancer prevention. The recommendation is not yet finalized and is currently open for public comment and further review.
Cervical cancer is a malignancy that arises in the cells of the cervix, the lower part of the uterus that connects to the vagina. According to the World Health Organization (WHO), it is the fourth most common cancer in women worldwide, with a significant portion of cases linked to persistent infection with high-risk types of HPV. HPV is a common sexually transmitted infection, and while most infections clear up on their own, persistent infections with certain high-risk types can lead to the development of cervical abnormalities and, ultimately, cancer.
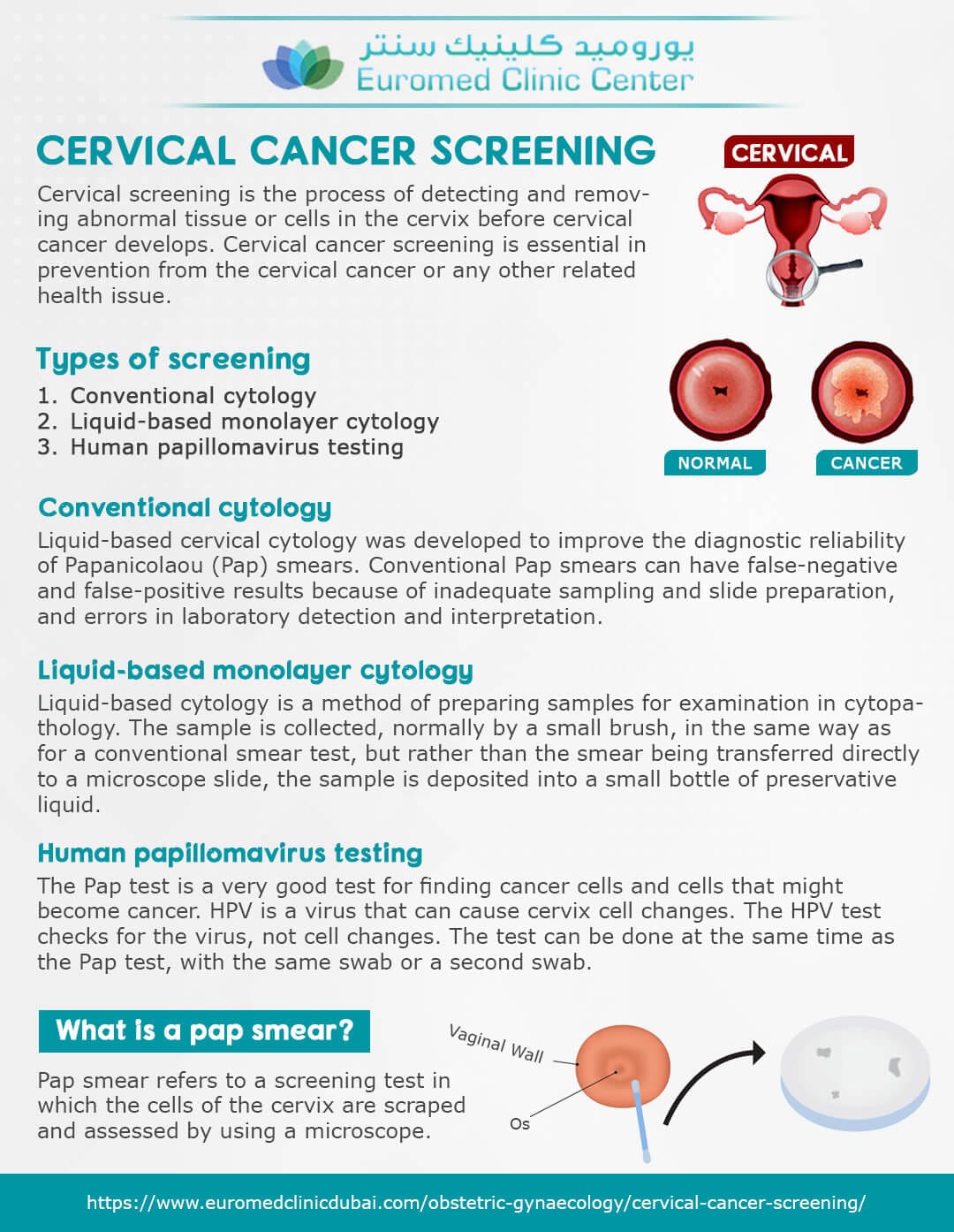
The current screening guidelines typically recommend Pap tests, which examine cells scraped from the cervix for abnormalities that could indicate pre-cancerous changes. Pap testing has been instrumental in reducing cervical cancer rates over the past decades. However, it is known that the Pap test is not perfect, and can occasionally miss abnormalities. It also can occasionally produce false positive results, leading to unnecessary anxiety and follow-up procedures.
The rationale behind the proposed shift toward primary HPV testing stems from the understanding of the critical role HPV plays in cervical cancer development. An HPV test looks for the presence of the high-risk types of the virus, not changes in cervical cells. Studies suggest that HPV testing is more sensitive than Pap tests at detecting precancerous changes, particularly in women aged 30 and over. This heightened sensitivity allows for the identification of women at higher risk of developing cervical cancer much earlier in the disease process.
The recommendation specifically targets women 30 years and older because it’s among that age group that HPV infection is more likely to become persistent. Younger women are more likely to clear HPV infections naturally, and that can cause unnecessary interventions. By focusing on those 30 and older, the task force hopes to maximize the effectiveness of screening while minimizing the potential for over-testing and unnecessary procedures. For women under the age of 30, Pap tests may continue to be used, and HPV testing can be added for women with abnormal results on a Pap test.
If the new recommendations are adopted, the process would likely involve a woman’s doctor taking a sample of cervical cells, which would be sent to a laboratory for analysis. The laboratory would then determine if any high-risk HPV types are present. If the HPV test is negative, then follow up screening would be done less frequently, with the schedule depending on risk factors. If high-risk HPV types are present, then the patient will have a more in-depth evaluation, which may include a Pap test, a colposcopy (an examination of the cervix using a magnifying instrument), and potentially a biopsy.
It’s important to emphasize that the proposed change is not a complete dismissal of Pap testing. Pap tests still have value and may be used alongside HPV testing in certain circumstances. For example, if a patient has a positive HPV test, a Pap test will be added to evaluate the severity of the potential cervical abnormalities. Also, if HPV testing is not available for whatever reason, then Pap testing may be used for primary screening. In addition, some patients may still be screened with Pap tests based on the recommendation of their healthcare provider.
The task force emphasizes that the decision to shift primarily to HPV testing is based on a review of available scientific evidence. Research comparing HPV testing to Pap testing suggests that HPV testing has the ability to detect pre-cancerous changes that Pap testing might miss. This increased sensitivity can lead to earlier detection and intervention, potentially preventing the progression of the disease. Furthermore, HPV testing can be performed at the same visit as a Pap test, which adds efficiency for healthcare providers.
The potential benefits of the new screening protocol are numerous. Earlier detection of precancerous changes can prevent cervical cancer with relatively simple interventions. This could mean reduced rates of cervical cancer, fewer deaths attributed to the disease, and less invasive treatments needed, which in turn can lead to a lower burden of the disease.
Despite the potential benefits, there are some considerations that need to be taken into account if the new screening protocol is to be adopted. Access to HPV testing may be an issue, particularly in resource-limited areas or for individuals who may have limited access to healthcare. The cost of HPV testing could also be a deterrent, particularly since it is currently more expensive than Pap testing. Also, implementation of these new protocols will require time to train healthcare professionals and inform the public of the changes.
There may also be some challenges associated with public understanding and acceptance of this change. Many people are familiar with the Pap smear and have relied on it for many years. A widespread information and education program would be needed to ensure that individuals are aware of the changes and understand the reasons for the changes.
The task force is currently seeking comments from the public and from healthcare professionals on the draft recommendations. This period of public comment provides an opportunity for interested parties to share feedback and offer suggestions on the proposed changes. After considering the feedback, the task force will issue a final recommendation, which will be influential in shaping future cervical cancer screening guidelines.
As the draft guidelines are being discussed, it’s important for women to talk to their healthcare providers about what type of cervical cancer screening is best for them. The recommended screening methods can change from time to time, so it is best to get the most up-to-date information. It is also important to understand that screening is not a perfect method for detecting cancer. Screening should be combined with other measures, such as HPV vaccination, to achieve the best results.
The new recommendation is part of a broader effort to improve public health by using the latest advancements in medical technology and knowledge. The shift to primary HPV testing represents a significant step in cervical cancer prevention and management. It has the potential to save lives and greatly reduce the burden of this cancer worldwide.